Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
$ 20.50 · 4.7 (635) · In stock

Ideal Gases & Real Gases, PDF, Gases

Properties of gases extended oct 2020
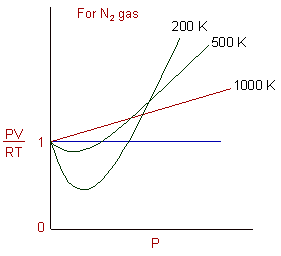
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

Compressibility Factor of Gas Overview, Equation & Chart

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts
Essential Pharma Documents: 1205: Properties of Gases
Gas compressibility factor Z: Ideal gas vs Real gas

Solved] The compressibility factor for an ideal gas is

The compressibility factor of a gas is defined as Z=P V / R T. The

OneClass: For a real gas, the compressibility factor, Z, is defined as Z (T, P) = PV/nRT For an ideal

