How serious is FDA warning about revolutionary blood-cancer
$ 15.99 · 4.6 (249) · In stock
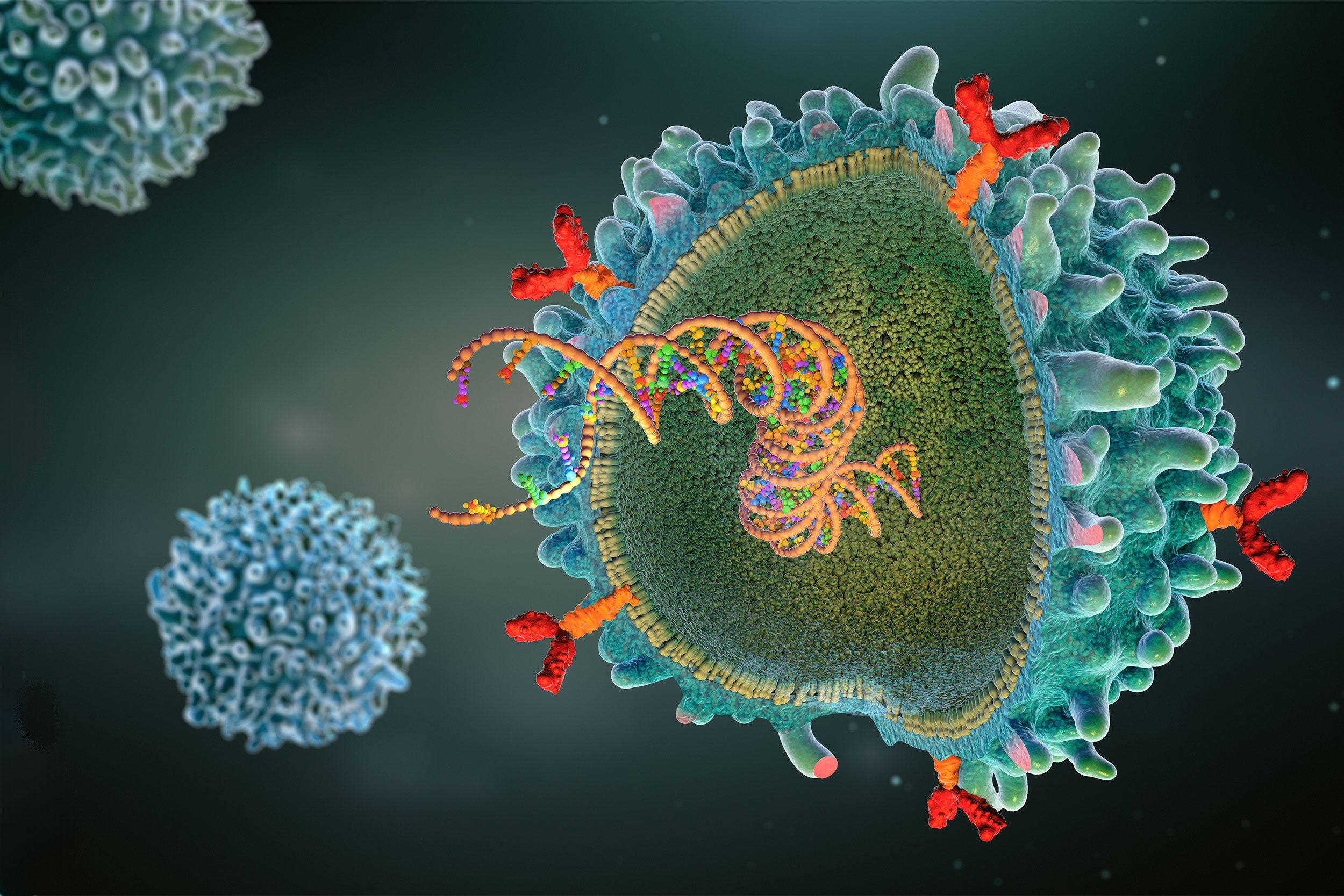
Dana-Farber Cancer Institute researcher details promise, peril of CAR T-cell therapy, which enlists body’s immune system to fight disease.
/medriva/media/media_files/7c7e91be7be56f903bf4aadf937ecff9c100ae250c9992bc7df5be0b75710906.jpg)
FDA Issues Stark Warning Against Unapproved Smartwatches and Rings for Blood Glucose Monitoring
James Wang, PhD on LinkedIn: Porton Advanced and Ascle Therapeutics Reached a Strategic Partnership to…
Mark Flower on LinkedIn: Updated: FDA approves Sarepta's Duchenne muscular dystrophy gene therapy…

At $475,000, is Novartis' Kymriah a bargain—or another example of skyrocketing prices?

FDA issues urgent warning as cancer treatment may actually increase cancer risk - Health - News - Daily Express US

Skipping Adjuvant Radiotherapy May Not Impact Risk of Recurrence or Progression in Patients with Low-Risk DCIS - Be part of the knowledge - ReachMD

Archives — Page 5 of 875 — Harvard Gazette
James Wang, PhD on LinkedIn: Bispecific CAR T-Cell Therapy Has “Robust” Activity in Non-Hodgkin Lymphoma

FDA puts Gilead's blood cancer drug studies on hold

FDA announces first US gene therapy approval for cancer treatment
Eric Smith (@ESmithMDPhD) / X
Mark Flower on LinkedIn: Julie Kanter, MD, on Preparing for Anticipated Approval of Lovo-Cel for…
ICancer Event

Eric Smith (@ESmithMDPhD) / X





