Why do pressure and temperature increase during the compression of
$ 11.50 · 4.8 (597) · In stock

The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in this article.

adiabatic-reversible-expansion-and-compression-summary - LearnChemE
Example Problem with Otto Cycle

Why does water boil faster at high altitudes? - tec-science
tec-science, Autor bei tec-science

Why does water boil faster at high altitudes? - tec-science
Heat Transfer and Applied Thermodynamics: Compression Heating of a Gas

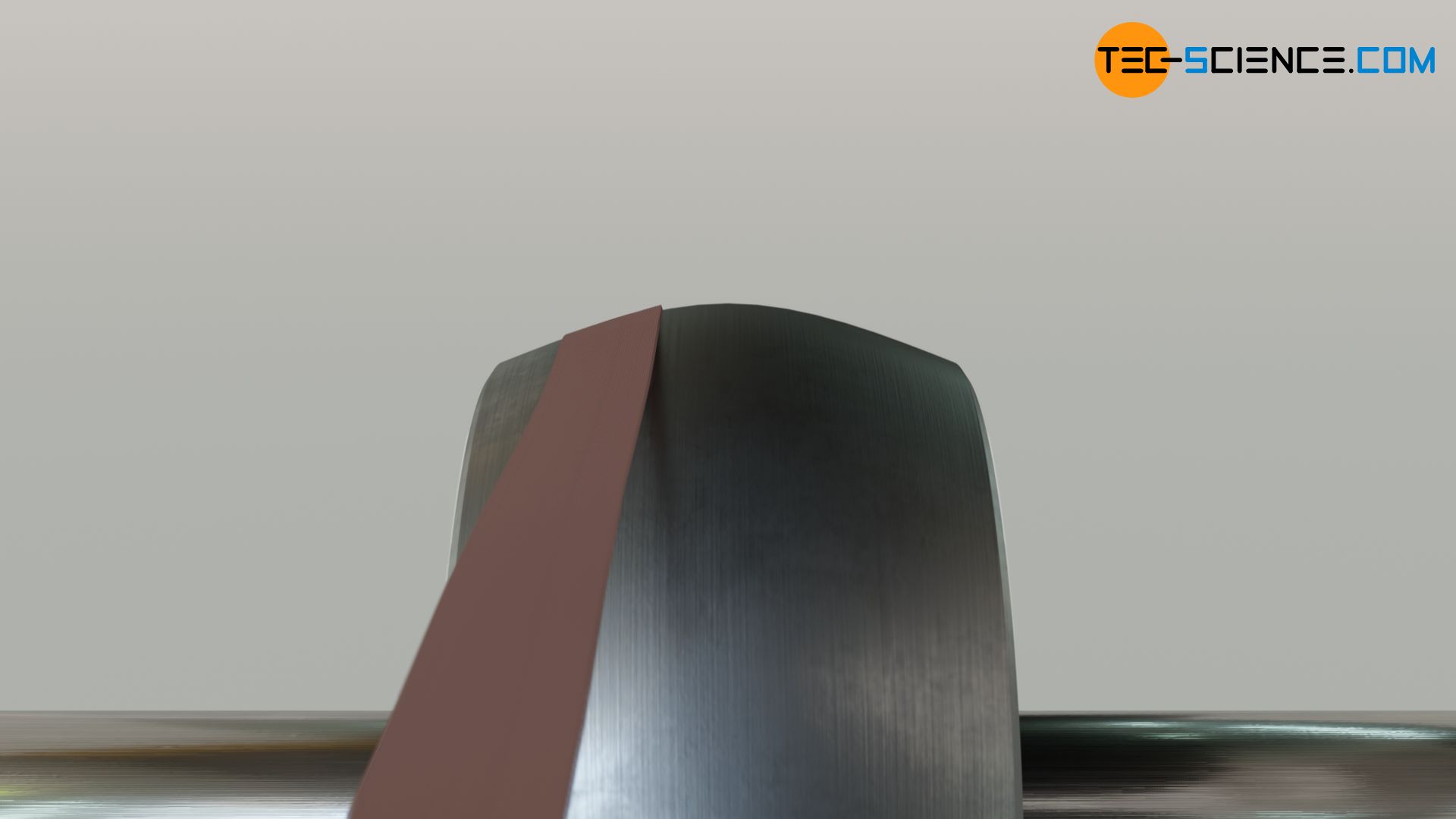
Air temperature rise during compression

Air at 100 KPa and 280 K is compressed steadily to 600 KPa and 400
Thermodynamics Archive - tec-science
Solved The volume of a gas is halved during an adiabatic

Difference between latent heat of vaporization and enthalpy of vaporization - tec-science

Boyle's law states that when a sample of gas is compressed at a
