Calculate the number of molecules of CO_2 present in 4.4 g of it.
$ 14.50 · 4.5 (409) · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:calculate the number of molecules of co2 present in 44 g of it
Click here👆to get an answer to your question ✍️ Calculate the number of molecules of CO-2 present in 4-4 g of it

Calculate the mass of carbon present in 2 g of carbon dioxide. (a) 128 (b) 6 g 0.545 g (d) 5.45 g
How many number of moles are present in 24.088 x 10²³ numbers of sodium atoms? - Quora

Calculate the ratio of molecules present in 6.6g of {CO}_{2} and 3.2g of sulphur dioxide.

Calculate the number of molecules present in 4.4g of CO2. [At Mass: C=12, 0=16 u,NA = 6.02 x1023 mol

Calculate mass of so2 gas which will contain same number molecules present in 4.4g of co2
What is the mass of three moles of carbon dioxide? - Quora

Calculate (i) number of molecules present in 2.24 dm^3 of carbon dioxi
How many grams of SO2 are present in 0.4 moles of SO2? - Quora
31. which of the folowing has lowest weight? 1) 6.023 x 1022 molecules of glucose 2) 18ml of water at 40C 3) 11200ml of CH4 at stp 4) 5.6 litre of CO2 at stp

The number of oxygen atoms in 4.4 g of CO2 is approx 6x 10 (c) 6 x 1023 (d) 12 x 1023 (b) 1.2 x 1023
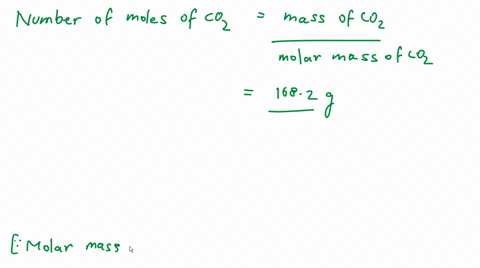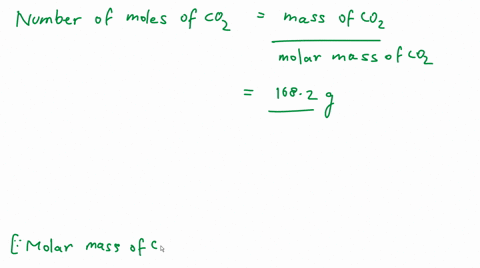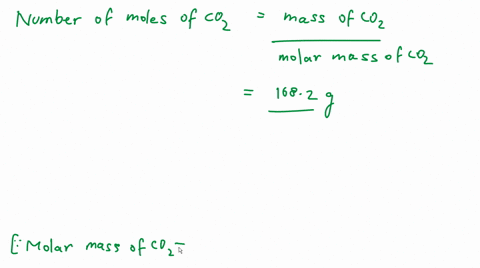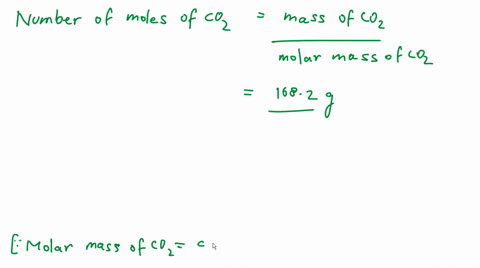
SOLVED: 'calculate the number of molecules present in 4.4 g of co2

Calculate mass of Nitrogen (N2) which contains same number of molecules as are present in 4.4g of carbon
2.24 l co2 gas at ntp find mass
Solved Calculate the number of molecules present in each of

Carbon monoxide - Wikipedia