Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an
$ 11.00 · 4.8 (710) · In stock

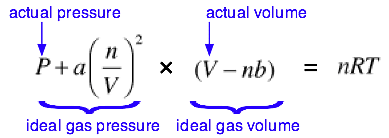
Derivation of Van Der Waals Equation: Real & One Mole of Gas

What is compressibility factor? What is its value for ideal gas

Thermodynamics: An Engineering Approach - 5th Edition - Part II by 黑傑克 - Issuu
Gas compressibility factor Z: Ideal gas vs Real gas

physical chemistry - Pressure vs volume plot for real gas and ideal gas - Chemistry Stack Exchange
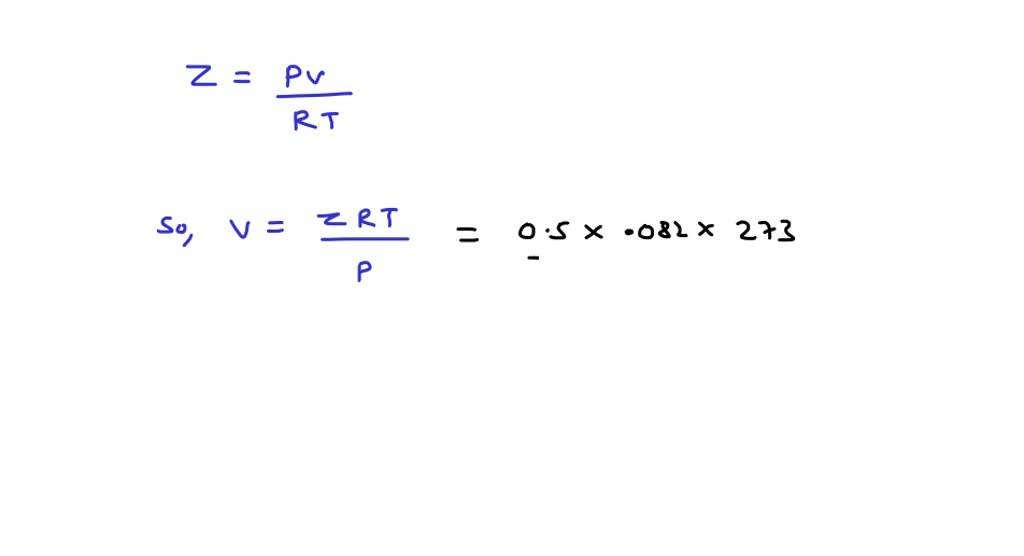
⏩SOLVED:Compressibility factor for 1 mol of a van der Waals gas at…

Solved] The compressibility factor for an ideal gas is

The compressibility factor (Z) of one mole of a van der Waals' gas of negligible 'a ' value is:1dfrac{bp}{RT}1+dfrac{bp}{RT}1-dfrac{bp}{RT}

If Z is a compressibility factor, vander Waals equation low pressure can be written as [JEEN (0)2=1 Rang (1) Z= 1 + RT Pb (2) Z 2)2=1= = 1 - 2= (3) Z = 1 - 42=1 (4)Z = 1 + VRT

Real-gas z-factor, as attributed to Standing and Katz, 9 plotted as a

Solved We begin by showing that the compressibility factor

Real-gas z-factor, as attributed to Standing and Katz, 9 plotted as a

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
