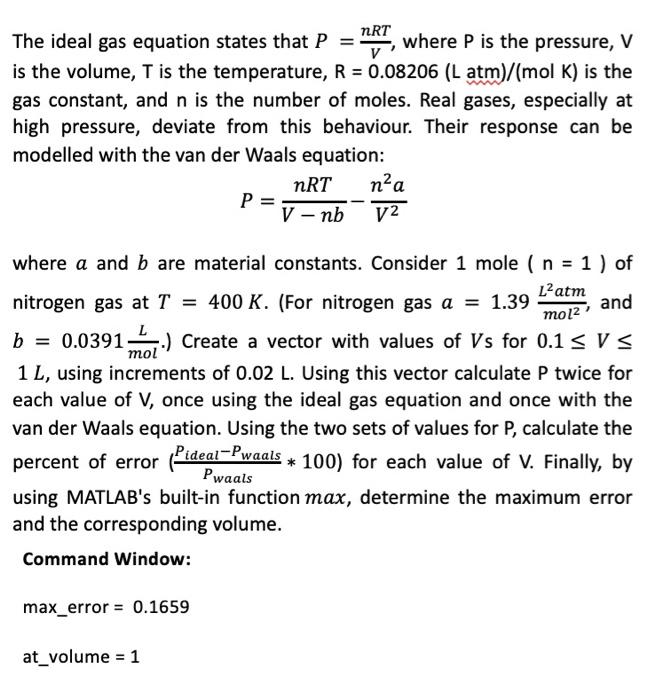
Solved) - NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, (1 Answer)
$ 15.50 · 4.6 (110) · In stock
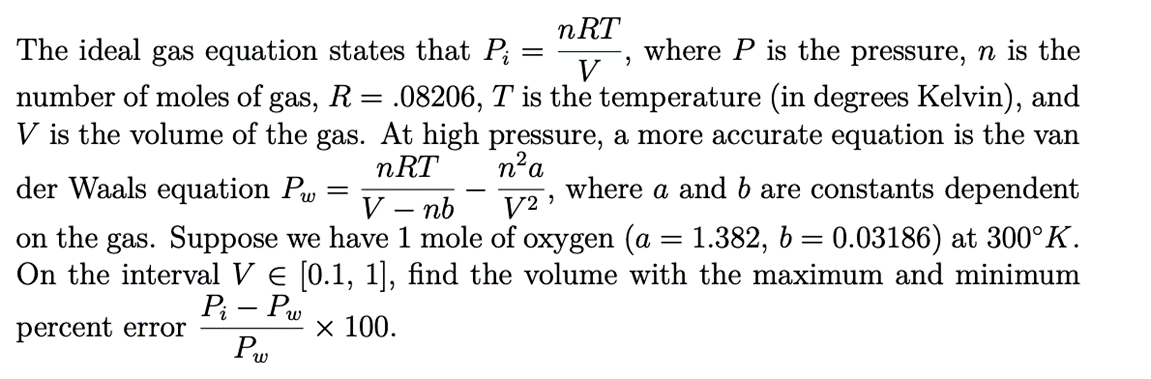
amp;#160;NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, N Is The V Number Of Moles Of Gas, R= .08206, T Is The Temperature (In Degrees Kelvin), And V Is The Volume Of The Gas. At High Pressure, A More Accurate Equation Is The Van NRT

The graph below shows the change in pressure as the temperature i

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

Ideal Gas Law - an overview

Ideal Gas Law Practice Problems
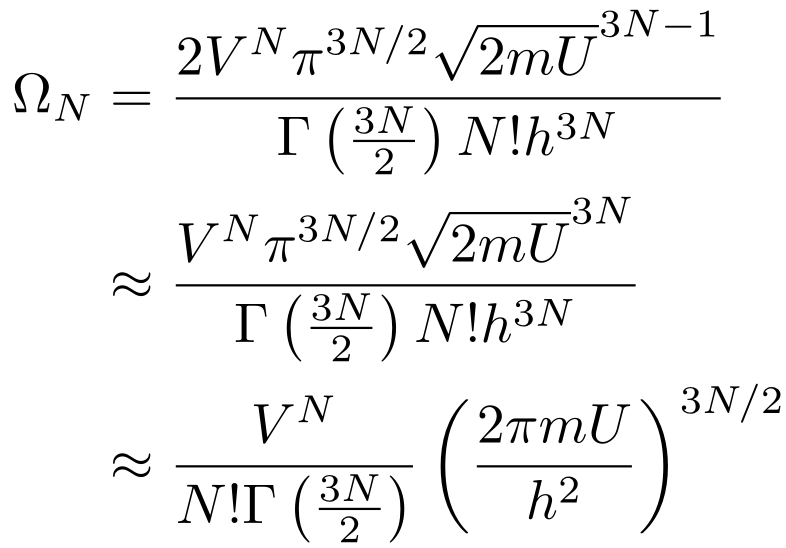
Let's Derive the Ideal Gas Law from Scratch!
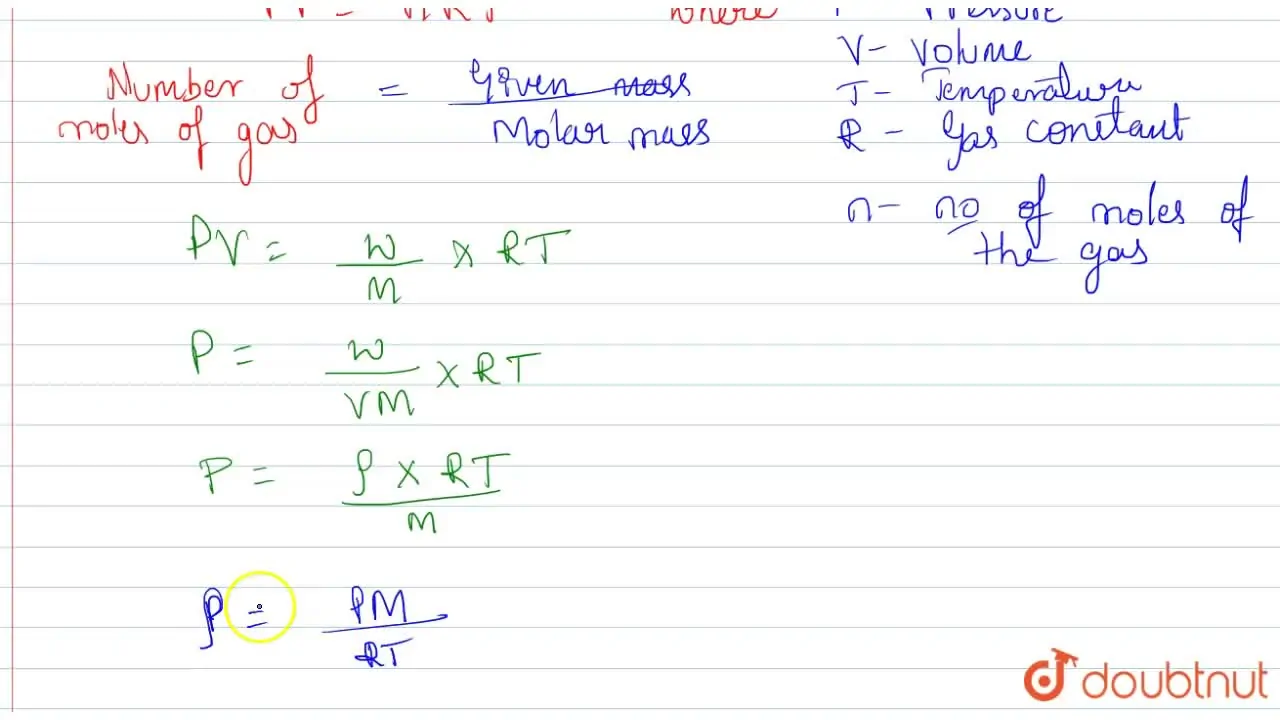
Kannada] Derive the relation between Density and Molar mass of a gase

Ideal Gas Law - an overview
Understanding the Fundamental Gas Laws: The Ideal Gas Equation

OpenStax College Physics, Chapter 13, Problem 24 (Problems
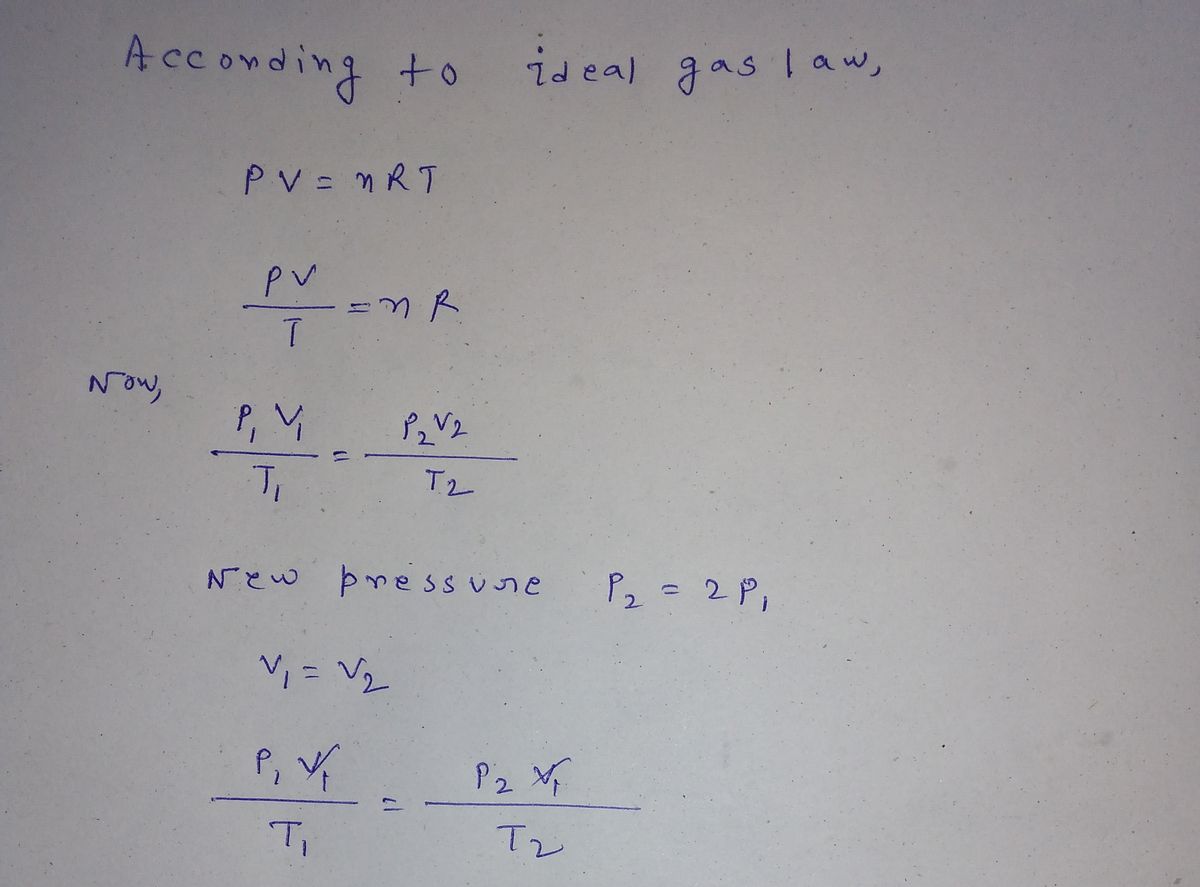
Answered: Consider an ideal gas with an absolute…
Solved NRT = The ideal gas equation states that P = no?